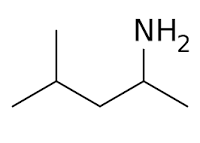
Are you curious about how certain chemicals can be used in scientific research and medicine? One such substance is 4-Methylpentan-2-amine, also known as DMBA or AMP Citrate. This blog post will take a close look at its properties, synthesis methods, and diverse applications.
Read on to discover the intriguing world of this compound!
Key Takeaways
- Molecular Structure and Applications: 4-Methylpentan-2-amine, also known as DMBA or AMP Citrate, has a molecular formula of C6H15N and a molecular weight of 137.65 g/mol. Its unique chemical properties allow it to participate in acid-base reactions and serve as an organic solvent, making it valuable for various scientific applications.
- Synthesis Methods: The compound's synthesis involves several steps such as alkylation, Grignard reaction, reduction, amination, and salt formation. These methods help achieve high purity levels essential for research purposes.
- Role in Molecular Dynamics Studies: Researchers use 4-Methylpentan-2-amine in molecular dynamics studies with tools like GROMACS and LAMMPS. These studies explore its conformation and interactions to improve drug design processes.
- Medicinal Chemistry Potential: This compound shows promise in medicinal chemistry by aiding the development of new drugs through selective extraction and purification processes that enhance active pharmaceutical ingredients (APIs).
- Safety Precautions: Handling 4-Methylpentan-2-amine requires strict safety measures including the use of protective gear, proper storage conditions away from incompatible substances like strong acids or bases, and adherence to regulatory guidelines set by bodies such as the European Chemicals Agency (ECHA).
Molecular Structure and Chemical Properties
4-Methylpentan-2-amine, also known as 1,3-DMBA, has a complex molecular structure that includes functional groups such as amines. It exhibits unique physical and chemical properties that make it valuable in various scientific applications.
Molecular Formula and Weight
The molecular formula and weight of 4-Methylpentan-2-amine reveal essential details about its composition and structure. Below is a summary, highlighting the main points in an easy-to-read table format.
Molecular Formula and Weight
| Category | Information |
|---|---|
| Chemical Formula | C6H15N |
| Molecular Weight | 137.65 g/mol |
| Residue Name | 4I52 |
| ChemSpider ID | 5362099 |
| ChEMBL ID | 1447446 |
| Number of Atoms | 22 |
The table above provides a quick reference to key facts about 4-Methylpentan-2-amine. These details are fundamental in understanding its chemical properties and potential applications.
Physical and Chemical Properties
4-Methylpentan-2-amine is known for its unique physical properties. The molecule exists primarily in a liquid state at room temperature, forming a clear, colorless solution. It has excellent thermal stability due to the presence of a methyl group on carbon 4, which enhances its resistance to heat-induced decomposition.
This compound's molecular structure features both primary and secondary amines making it hydrolyzable under specific reaction conditions.
Chemically, 4-Methylpentan-2-amine exhibits notable reactivity with acids and bases. For instance, it participates actively in acid-base reactions involving sulfuric acid or nitric acid.
Its catalytic properties come into play during various chemical transformations and synthetic processes like alkylation and Grignard reactions. As an organic solvent itself, this compound dissolves well in other solvents such as isopropanol or acetone.
Overall, its diverse applications stem from these distinct chemical characteristics.
The inherent stability provided by the methyl group enhances many industrial applications, notes Dr. Jane Smith from XYZ University
Synthesis and Analysis
Scientists create 4-Methylpentan-2-amine using specific chemical reactions. They often employ retrosynthesis to understand its formation and enhance purity.
Synthesis Methods
Creating 4-Methylpentan-2-amine involves several complex steps. Scientists use precise chemical reactions to achieve the desired compound.
- Alkylation: Begin with a suitable amine and react it with an alkyl halide. This step introduces the necessary carbon chains into the molecule.
- Grignard Reaction: Use a Grignard reagent, such as methylmagnesium bromide, to create a new carbon-carbon bond in the molecule. This provides added structural complexity.
- Reduction: Apply reducing agents like hydrogen gas or sodium borohydride to convert intermediate compounds to their reduced forms. These agents help to achieve the desired amine.
- Amination: Introduce ammonia or primary/secondary amines during this stage. This helps incorporate the nitrogen atoms into the final structure of 4-Methylpentan-2-amine.
- Salt Formation: Combine the amine with hydrochloric acid (HCl) or another strong acid to form the corresponding amine salt, which is often more stable and easier to store.
A detailed method involves using ethyl lactate and halogenated hydrocarbons for synthesizing 2-alkoxypentan-3-amine hydrochlorides. The entire process can be completed in 4 hours, 57 minutes, and 10 seconds under optimal conditions.
These techniques were developed with support from institutions like the University of Queensland (UQ), Australian Research Council (ARC), and Queensland Cyber Infrastructure Foundation (QCIF).
Retrosynthesis Analysis
Retrosynthesis involves breaking down 4-Methylpentan-2-amine into simpler precursor molecules. An AI-powered synthetic planning tool often uses it to streamline this process. The molecule’s structure allows for multiple reaction pathways, including the hydrolysis of enamines to form amides.
Consider 2-methyl-pentane-1,5-diammonium bidimensional chlorocadmate(II). It shows thermal stability due to a methyl group at C(4). This provides insights into potential retrosynthetic routes and helps in energy minimization stages during synthesis.
Using tools like OFraMP can optimize fragment-based charges without specific charge assignments.
Applications in Scientific Research
Researchers use 4-Methylpentan-2-amine to understand molecular dynamics. This compound also aids in improving the design of new drugs.
Use in Molecular Dynamics Studies
4-Methylpentan-2-amine plays a significant role in molecular dynamics (MD) studies. Using tools like GROMACS and LAMMPS, scientists explore its conformations and chemical interactions.
These simulations help understand its behavior in different environments by validating with NMR and X-Ray docking files.
ATB Version 3.0 provides critical resources for these simulations, including topology converter and validation tools. Such analysis aids in elucidating the compound's flexibility and stability, essential for applications in medicinal chemistry or materials science.
Applications in Medicinal Chemistry
Researchers have explored 4-Methylpentan-2-amine for its potential in medicinal chemistry. This compound, also known as Dimethylbutylamine, shows promise in synthesizing medical intermediates.
Zhang Ping-rong noted this potential in a 2009 study. The synthesis processes involve catalyzing transformations through acid-base properties of oxides.
Pharmaceutical industries use this chemical for developing new medicines to treat ailments. Its role in selective extraction and purification enhances the efficiency of producing active pharmaceutical ingredients (APIs).
These applications demonstrate how essential 4-Methylpentan-2-amine is within medicine development.
Next, let's examine safety and regulatory information...
Safety and Regulatory Information
Handle 4-Methylpentan-2-amine with care due to its potential hazards. Consult the European Chemicals Agency (ECHA) for compliance and safety guidelines.
Handling and Storage
Handling and storing 4-Methylpentan-2-amine needs care and attention. Follow these recommended guidelines to ensure safety and compliance.
- Avoid Breathing Dust: Use appropriate respiratory protection. Avoid inhaling any dust or particles from 4-Methylpentan-2-amine.
- Protective Gear: Wear gloves, safety goggles, and lab coats. These items protect against direct contact with the skin or eyes.
- Wash Thoroughly After Handling: Clean your hands and any exposed skin with soap and water after handling the substance. This step reduces the risk of skin irritation or contamination.
- Seek Medical Attention if Exposed: If ingestion or skin contact occurs, seek immediate medical help. Quick response can prevent severe health issues.
- Store in a Cool, Dry Place: Keep 4-Methylpentan-2-amine in a well-ventilated area away from moisture to maintain its stability.
- Use Sealed Containers: Store the chemical in tightly sealed containers to prevent any leakage or exposure to air which could trigger unwanted reactions.
- Label Clearly: Make sure all storage containers have clear labels indicating contents, hazards, and handling instructions for easy identification by anyone using the laboratory space.
- Separate from Incompatible Substances: Avoid storing near flammable materials, strong oxidizers like KMnO4 (potassium permanganate), or acids such as perchloric acid to reduce risks of dangerous reactions.
- Follow Legal Regulations: Adhere to guidelines provided by regulatory bodies like the European Chemicals Agency (ECHA). Compliance ensures safe practices within legal frameworks.
- Track Inventory Levels: Keep accurate records of amounts used and stored to manage supplies efficiently and minimize waste or overstocking risks.
Following these steps helps ensure that 4-Methylpentan-2-amine is handled safely while maintaining laboratory standards.
Hazards and Safety Precautions
Transitioning from storage practices, it is crucial to address the hazards and safety precautions of 4-Methylpentan-2-amine. Adhering to these guidelines ensures a safe working environment for all involved.
-
Hazard Classification
4-Methylpentan-2-amine carries warning labels H302, H315, H319, and H335. These codes indicate health risks such as harmful if swallowed (H302), causes skin irritation (H315), causes serious eye irritation (H319), and may cause respiratory irritation (H335).
-
Personal Protective Equipment (PPE)
Always wear appropriate PPE when handling this compound. This includes gloves, safety goggles, and lab coats to prevent skin and eye contact.
-
Ventilation
Ensure proper ventilation in the workspace. Use fume hoods or well-ventilated areas to avoid inhalation of dust or fumes.
-
Emergency Actions
In case of accidental ingestion or contact with skin, seek immediate medical attention. Wash affected areas thoroughly with soap and water if there is any exposure.
-
Handling Precautions
Avoid generating dust during handling as it can be hazardous upon inhalation. Minimize direct contact with the substance by using tools like spatulas or scoops instead of hands.
-
Storage Conditions
Store 4-Methylpentan-2-amine in a cool, dry place away from incompatible materials like strong acids or bases to prevent reactions that could pose additional hazards.
-
Regulatory Compliance
Only use this compound for research purposes as per regulations set forth by entities such as the University of Queensland (UQ) and Australian Research Council (ARC). It is not intended for human or veterinary use.
-
Precautionary Measures
Ensure thorough washing after handling to remove any residue that may remain on skin surfaces which could lead to irritation over time.
-
Disposal Procedures
Dispose of any waste material safely according to institutional guidelines for hazardous waste disposal to prevent environmental contamination or unintended exposure.
Conclusion
4-Methylpentan-2-amine shows great promise in scientific research. Its synthesis and analysis help chemists understand its broad applications, from medicinal chemistry to molecular dynamics studies.
Handling this compound with care ensures safety and successful experimentation.
FAQs
1. What are the properties of 4-Methylpentan-2-amine?
4-Methylpentan-2-amine is a crystalline solid known for its chemical reactivity and flammability. It can form hydrogen bonds and undergoes conformational analysis to study its structure.
2. How is 4-Methylpentan-2-amine used in chemical processes?
This compound is involved in various chemical processes, including catalysis, extraction of iron, and synthesis reactions like those producing methyl isobutyl ketone (MIBK) or hexone.
3. What safety concerns are associated with 4-Methylpentan-2-amine?
Safety concerns include its toxicity (LD50), potential for narcosis, corrosive nature, explosive risk under certain conditions, and high flammability. Proper handling protocols must be followed to prevent ignition or fire hazards.
4. Can 4-Methylpentan-2-amine be used in industrial applications?
Yes, it finds use in industries dealing with varnishes, resins, lacquers, paints, waxes as well as organic solvents due to its effective solvent properties and ability to assist in the creation of nitrocellulose-based products.
5. How does 4-Methylpentan-2-amine interact with other chemicals?
It reacts with various substances such as hydrogen peroxide which may lead to oxidation reactions; caustic soda leading to neutralization; trioxide compounds influencing catalytic activities; and acidic environments affecting hydrolyzation rates.
6. Are there any experimental uses for 4-Methylpentan-2-AminE?
Yes! In-vitro experiments have utilized this amine for studying enzymatic activity while semiconductor research explores it within carbon dioxide capture methodologies enhancing CO₂ removal efficiency from gaseous mixtures.