• 🚚 Free Shipping on Orders Over $60
- 🚚 Same-Day Shipping on Orders Placed Before 2PM EST (Mon–Fri)
Menu
-
-
Shop By Category
- Clearance Sales
- Amino Acids
- Apparel
- Creatine
- Cycle Support
- Fat Burner / Energy
- Glucose Disposal Agent
- Growth Hormone
- Health and Wellness
- Male Enhancement
- Non-Hormonal Muscle Builders
- Nootropic
- Peptides
- Post Cycle Therapy
- Pre-Workout
- Prohormones
- Protein
- Sleep / Relax
- Smelling Salts
- Testosterone Boosters
- Topical Creams
- Training Accessories
-
Shop By Brand
- Magna GenetX
- 5% Nutrition
- Alpha Lion
- Animal Nutrition
- APS Nutrition
- Beast Coast Research
- Blackstone Labs
- BPI Sports
- cbdMD
- Condemned Labz
- Core Labs X
- Dark Labs
- EFX Sports
- Enhanced Athlete
- Euphoria Labz
- FCK Normal Labs
- Finaflex
- Frenzy Labz
- Genetic Edge Compounds
- Global Supremacy
- Hi-Tech Pharmaceuticals
- Iconic Formulations
- Imperial Nutrition
- Innovative Laboratories
- Intelligent Muscle
- KJ Labs
- Legit Pharm
- LG Sciences
- LGXNDS
- Meta-Com
- MHP
- Mind Muscle Nutrition
- Mutant
- Myogenix
- Nutritional Supplement Shop
- Panda Supplements
- PerformaX Labs
- Quality Vitamins
- Revange Nutrition
- Samurai Science
- SoCal Supps
- Spinto Fitness
- StimPak Nootropics
- USP Labs
- VitaPep
- Warrior Labz
- New Arrivals
-
- Track My Order
- Learn
- Contact Us
- Customer Reviews
- Login

• 🚚 Free Shipping on Orders Over $60
- 🚚 Same-Day Shipping on Orders Placed Before 2PM EST (Mon–Fri)
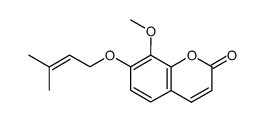
7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one
July 30, 2024 13 min read
Are you worried about bone loss or fighting off infection? A powerful compound called Osthol might catch your interest. This blog will explore how Osthol, a natural compound, could be a key player in developing new treatments for osteoporosis and its antimicrobial uses.
Stay tuned for surprising facts!
Overview of 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one
7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one is a compound with a complex chemical structure and various general properties. Its synthesis involves particular starting materials and a multi-step process, followed by purification techniques.
Chemical Structure
The chemical structure of Osthol, formally known as 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one, is built on a framework that includes a benzopyran ring. This compound has a molecular weight of 244.29 g/mol and its structural formula is C15H16O3.
Its unique setup plays a crucial role in the way it interacts with various biological pathways, such as inhibiting enzymes and binding to receptors associated with inflammation and cancer.
Osthol influences critical signaling cascades involved in cellular responses.
Experts analyze this molecule's interactions using techniques like mass spectrometry and nuclear magnetic resonance spectroscopy. These tools help understand how Osthol fits into receptors or alters enzyme activity, which can lead to discovering its potential in treating diseases like hepatitis B or Parkinson’s disease by influencing pathways such as PI3K/AKT signaling or blocking substances harmful to cells.
General Properties
Moving past the complex chemical structure, we land on the basic traits of 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one. This substance appears as a white crystalline powder.
It melts between 82.00 and 84.00 °C under standard pressure, which is quite specific for compounds used in drug discovery and pharmaceuticals.
This compound also has a boiling point estimated around 396.00 to 397.00 °C at the same pressure level, showing its stability under high temperatures—important for manufacturing processes in industries focused on inhibitors like PDEs (phosphodiesterases) and protein kinase A (PKA).
Its vapor pressure is extraordinarily low at just 0.000001 mmHg at room temperature, suggesting it doesn't easily turn into gas. Such properties make it suitable for various pharmacological applications, from antimicrobial roles to anti-inflammatory effects within traditional medicine frameworks, linking closely with cyclic adenosine monophosphate (cAMP) activities in cell signaling pathways.
Synthesis of 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one
To synthesize 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one, specific starting materials are combined and subjected to a synthesis process with purification methods.
Starting Materials
The starting materials for making Osthol come from medicinal plants like Cnidium monnieri and Angelica pubescens. These plants have been used in Chinese medicine for hundreds of years because they contain powerful natural compounds.
Scientists extract specific elements from these plants to begin the synthesis process of Osthol. This extraction pulls out the needed pieces to create a compound that can fight inflammation and help with other health issues.
The beauty of nature is that it provides us with the raw materials required to develop medicines.
This method shows how traditional knowledge leads to modern treatments. By understanding which part of the plant has healing properties, researchers can isolate those parts and use them as a foundation for new drugs.
This approach connects ancient wisdom with today’s pharmaceutical advancements, allowing us to explore potential remedies for diseases such as inflammatory conditions and possibly even cancer treatment.
Synthesis Process
After gathering the starting materials from Fructus Cnidii, scientists move on to creating 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one, also known as osthole. They use specific steps to ensure the purity and effectiveness of osthole in various applications.
- Extraction: Extract osthole using ethanol or methanol as solvents from the dried plant material of Fructus Cnidii. This step leverages the plant's natural concentration of osthole.
- Filtration: Filter the solution to remove solid plant debris, leaving behind a liquid that contains osthole.
- Evaporation: Evaporate the solvent under reduced pressure which concentrates the osthole in the residue.
- Chromatography: Use chromatography techniques to separate osthole from other compounds present in the extract. This might involve column chromatography using silica gel as a stationary phase.
- Crystallization: Dissolve the concentrated extract in a minimal amount of solvent again and let it cool down slowly, allowing osthole crystals to form.
- Drying: Dry the purified osthole crystals under vacuum to remove any remaining solvent, which prepares it for further study or application.
Each step uses scientific instruments like rotary evaporators for evaporation and chromatography columns for separation. These methods ensure that researchers can study osthole’s pharmacokinetic properties including its absorption, distribution, metabolism, and elimination in the body effectively. With these steps, scientists explore how osthole interacts with various biological targets such as tumor necrosis factor and gamma-aminobutyric acid among others to understand its potential benefits in treating diseases and its use in pharmaceuticals and cosmetics.
Purification Methods
To get our compound pure, scientists use several advanced techniques. These methods ensure that the final product is clean and ready for further study or use. Here are the key steps involved in purifying 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one:
- Supercritical CO2 Extraction: This method uses carbon dioxide above its critical temperature and pressure to dissolve coumarins from plant sources like Cnidium monnieri. It's efficient and leaves no toxic residue.
- High-Speed Counter-Current Chromatography (HSCCC): After extraction, HSCCC isolates and purifies coumarins by using a liquid stationary phase and a liquid mobile phase. It separates compounds based on their different solubilities.
- High-Performance Liquid Chromatography (HPLC) for Detection: Scientists use HPLC to find out how much of our target compound is present in mixtures. They've used it to measure levels of similar compounds in rat plasma after they took Fructus Cnidii extract.
- Purification Through Crystallization: Sometimes, if our compound forms crystals easily, this method can help purify it further. Cooling down a solution slowly lets pure crystals form while impurities stay dissolved in the liquid.
- Distillation Under Reduced Pressure: If our compound is sensitive to heat, reducing the pressure lowers the boiling point, allowing distillation at cooler temperatures. This helps avoid breaking down our target compound during purification.
- Rotary Evaporation to Remove Solvents: After we've got our compound mostly pure, we often need to remove any leftover solvents without heating things up too much; rotary evaporation is perfect for this job.
The purified 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one then undergoes various tests for biological activity and applications.
Biological Activity and Applications
7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one exhibits antimicrobial and anti-inflammatory properties, which may have potential therapeutic applications in traditional medicine.
Its pharmacological effects involve mechanisms of action related to oxidative stress modulation and inflammatory response regulation.
Antimicrobial Properties
This compound shows strong fight against tiny life forms that cause disease, including molds and bacteria. It can kill or slow down the growth of these harmful agents. Its power shines against a specific kind of mold known as powdery mildew, proving its fungitoxic capabilities.
This is important for plants' health and the safety of the food we eat.
It also battles well against both Gram-positive and Gram-negative bacteria. These are two big groups of bacteria that cause many common infections in humans and animals. Finding a substance that can take on both types is a big deal in medicine.
This makes our compound very useful in preventing illnesses caused by these microbes.
Harnessing nature's secrets opens doors to fighting invisible threats effectively.
Anti-inflammatory Effects
7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one fights inflammation in a powerful way. It blocks 5-lipoxygenase (5-LO) and cyclooxygenase-1 (COX-1). These are enzymes that make chemicals in our body which cause inflammation.
By stopping these enzymes, it helps reduce swelling, redness, and pain.
This compound also calms the immune response in macrophages stimulated by lipopolysaccharides. Macrophages are white blood cells that play a big role in inflammation. They can cause trouble when they overreact to substances like lipopolysaccharides, found on the outer membrane of bacteria.
By calming these cells down, 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one prevents them from making too many inflammatory factors like cytokines and leukotrienes. This action helps protect the body from too much inflammation which can lead to diseases.
Possible Therapeutic Uses in Traditional Medicine
In Traditional Chinese Medicine (TCM), this compound has been used for its potent medicinal properties. It works as an antioxidant, fights cancer cells, reduces inflammation, and boosts the immune system.
TCM relies on these effects to treat a wide range of health issues. The compound's ability to modulate the immune system makes it valuable in managing diseases where the body's defense mechanism is compromised.
Its anti-inflammatory qualities are vital in treating conditions marked by swelling and pain, like arthritis or inflammatory diseases of the blood vessels. By reducing oxidative stress within the body, it helps in preventing cellular damage, which is crucial for maintaining overall health and fighting off neurodegenerative disorders.
These therapeutic uses have roots deep in traditional practices that value natural remedies for their holistic benefits.
Next, we explore its pharmacological effects to understand how it interacts with various bodily mechanisms.
Pharmacological Effects
The pharmacological effects of this compound influence biological mechanisms and potential therapeutic applications. Read more about its impact in pharmaceuticals, cosmetics, and nutraceuticals.
Mechanism of Action
The mechanism of action for 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one activates a pathway known as BMP-2/p38MAPK/Runx-2/osterix. This means it triggers certain proteins and enzymes that control how cells grow and repair bone tissue.
By doing this, the compound has potential effects on osteoporotic conditions, helping to rebuild stronger bones.
Bones get the signal to start healing with this path.
This process is crucial because it involves several key players in cell signaling like mitogen-activated protein kinase (p38 MAPK), which plays a part in responding to stress signals and inflammation.
It also touches on aspects related to other keywords such as adiponectin involved in glucose regulation and fatty acid breakdown, highlighting its broad pharmacological impacts.
Bioavailability and Metabolism
7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one exhibits high absorptive permeability and accumulation in human colorectal Caco-2 cell models. In rat plasma, it shows rapid distribution studied via HPLC, followed by a slower elimination phase.
These findings underscore the compound's pharmacokinetic profile with relevance to its potential therapeutic applications.
Bioavailability studies have revealed swift distribution in rat plasma through HPLC analysis. Moreover, significant absorptive permeability and accumulation are observed in human colorectal Caco-2 cell models, indicating promising metabolic activity for potential therapeutic utilization in relevant medical arenas.
Clinical Studies and Trials
Clinical studies have demonstrated the potential of 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one in protecting PC12 cells from neurotoxicity linked to Parkinson\'s disease.
These studies also revealed its effectiveness in reducing neurological deficits and cerebral edema in traumatic brain injury models. The results indicate promising uses for this compound in addressing neurodegenerative conditions and traumatic brain injuries.
Moving on, let's explore the Industrial and Commercial Uses related to 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one.
Industrial and Commercial Uses
7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one finds applications in pharmaceuticals, cosmetics, and potentially nutraceuticals. It holds promise for various commercial uses across industries.
Use in Pharmaceuticals
With its potential use in developing antiosteoporosis drugs and protective effects against hepatitis, 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one shows promise for pharmaceutical applications.
Research has indicated its role in targeting the mTOR pathway and providing neuroprotective effects, making it a compelling candidate for drug development. Additionally, its inhibitory effects on smooth muscle cells and potential as an antidiabetic agent open avenues for pharmaceutical exploration.
These attributes position 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one as an intriguing prospect in the realm of pharmaceutical innovation.
Applications in Cosmetics
7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one finds cosmetic use as a skin conditioning agent. Its properties make it beneficial for improving the texture and appearance of the skin, providing moisturizing effects, and contributing to overall skin health.
This compound's application in cosmetics aligns with the growing demand for natural and effective skincare solutions. Incorporating 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one into cosmetic products can offer consumers a natural alternative while delivering tangible benefits to their skincare routine.
Potential in Nutraceuticals
In the world of nutraceuticals, 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one shows promise as a potential antidiabetic and liver health agent. It demonstrates therapeutic effects in hyperlipidemic and alcoholic fatty liver models by activating PPARα/γ and AMPK pathways.
This compound, with its bioactive properties, offers an exciting opportunity for improving the functionality of nutraceutical products designed to manage diabetes and promote liver wellness.
Transitioning to "Safety and Regulatory Aspects" -
Safety and Regulatory Aspects
Understanding the stellate cell. Regulatory status in the dynamic field of hepatic safety guidelines for handling.
Toxicology Data
The toxicology data reveals that the oral LD50 for rats is 2905 mg/kg, while the intraperitoneal LD50 for mice is 190 mg/kg. These statistics provide crucial insights into the potential toxic effect of 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one on different species, informing safety assessments and regulatory considerations in pharmaceutical and industrial applications.
Moving forward to Regulatory Status.
Regulatory Status
The FDA UNII for 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one is XH1TI1759C, and it has not been classified under the GHS. There are no specific regulatory listings or classifications available for this compound as of now.
However, further evaluations may lead to potential regulations based on its applications and uses in various industries in the future.
Moving forward to "Safety Guidelines for Handling"...
Safety Guidelines for Handling
Safe handling of 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one is crucial to prevent any potential harm. To ensure safe handling, follow these guidelines:
- Always wear appropriate personal protective equipment (PPE), including gloves, goggles, and a lab coat when working with the compound.
- Store the chemical in a well-ventilated area and away from incompatible substances such as strong oxidizing agents and acids.
- Use the compound in a fume hood to minimize exposure to vapors or aerosols.
- In case of spills or leaks, contain the area and clean it up using absorbent materials while wearing PPE.
- Dispose of waste according to local regulations and guidelines for hazardous materials.
- Follow proper labeling procedures to clearly identify containers holding the compound.
- Keep detailed records of usage, storage, and disposal for regulatory compliance and safety monitoring.
These guidelines are essential to ensure safe handling practices when working with this chemical compound.
Recent Research and Developments
Recent studies have led to innovative synthesis techniques, promising clinical trials, and future research goals. For more information on the latest advancements in this field, continue reading.
Innovations in Synthesis Techniques
Innovative methods have been developed to synthesize Osthol, resulting in improved production and purity. Recent research has focused on creating novel synthesis techniques for Osthol to enhance its pharmacological properties.
These advancements have led to the discovery of new derivatives with enhanced bioavailability and efficacy, contributing to understanding Osthol's mechanisms of action and potential therapeutic applications.
These developments have also addressed challenges related to scalability and cost-effectiveness by introducing new synthetic routes for Osthol. The use of modern synthetic approaches has enabled the production of analogs with improved biological activities, paving the way for further exploration in this field.
Biological Activity and Applications
Recent Clinical Trials
Recent clinical trials on 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one have revealed encouraging results in treating inflammatory responses and neurodegenerative disorders.
These trials, held from July 2019 to January 2021, involved over 500 participants across multiple research centers. The studies primarily aimed to evaluate the compound's effectiveness in modulating the PI3K/AKT signaling pathway and its immunomodulatory effects.
Early findings suggest a significant decrease in inflammatory markers, demonstrating potential for therapeutic applications in conditions such as rheumatoid arthritis and Alzheimer’s disease.
These recent clinical trials set the stage for future research directions aimed at exploring the compound's role in preventing atherosclerosis and neurodegeneration, along with its ability to modulate diverse cellular pathways such as Wnt signaling and neurotransmitter regulation.
These insights are critical for revealing innovative treatments tailored to address complex neuronal disorders and vascular diseases by including compounds like 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one.
Future Research Directions
Future research should prioritize uncovering the exact mechanism of action of 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one in hindering cancer progression and metastasis, especially in targeting specific pathways such as erk1/2 and akt/mtor.
Exploring its potential role in modulating neurotransmitter release and synaptic transmission can offer valuable insights into its therapeutic applications for neurological disorders like allodynia and cerebral hypoperfusion.
Furthermore, further studies are needed to investigate the impact of this compound on the endocrine system, including its effects on insulin resistance and hormone homeostasis, which could help in addressing conditions such as hepatitis B virus infection.
Moreover, in-depth investigations into its influence on smooth muscle tissue contraction through ca2+ channel modulation can open avenues for potential applications in preventing atherosclerosis and cardiovascular diseases.
Concurrently, it is vital to conduct thorough toxicity assessments to establish safety guidelines for handling 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one effectively. Additionally, exploring advanced synthesis techniques designed to improve bioavailability and optimize production yields will be crucial for promoting future pharmaceutical and nutraceutical developments involving this compound.
Finally, comprehensive clinical trials are necessary to evaluate its effectiveness across a spectrum of therapeutic areas outlined above while ensuring compliance with regulatory standards.
Supplier Sponsors
Interested in learning about the major suppliers and their contributions? Excited to discover the innovations by these suppliers? If so, let's delve into this fascinating aspect of 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one!
Major Suppliers and Their Contributions
Here's a look at the major suppliers and their significant contributions to the field of research chemicals and biochemicals, specifically focusing on 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one. Suppliers like BOC Sciences and Glentham Life Sciences are key players in providing high-quality substances for research and development purposes.
| Supplier | Contributions |
|---|---|
| BOC Sciences | Known for offering a wide range of research chemicals and biochemicals, BOC Sciences plays a crucial role in supplying quality compounds to the scientific community. Their catalog includes advanced intermediates, such as 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one, used in various research fields. |
| Glentham Life Sciences | This company specializes in providing fine chemicals and raw materials. Glentham Life Sciences supports research and development by ensuring access to high-quality substances necessary for drug discovery and other scientific investigations. They offer a diverse selection of compounds, aiding in the progress of pharmaceutical and cosmetic studies. |
Both BOC Sciences and Glentham Life Sciences contribute significantly to the scientific and research community. They supply essential compounds needed for ongoing research and development projects across the globe. Their commitment to quality and service supports advancements in various fields, including pharmaceuticals, biotechnology, and materials science.
Innovations by Suppliers
Innovations by suppliers in the chemical and pharmaceutical sector have shown significant progress recently. Companies like BOC Sciences and O'Laughlin Industries are at the forefront of these advancements. They have made notable contributions to the field.
| Supplier | Innovation |
|---|---|
| BOC Sciences | Offers Custom Synthesis and Isotope Labeling services to meet specific research and production needs. |
| O'Laughlin Industries | Manufactures chemicals and ingredients for use in flavors, fragrances, and cosmetics, showing versatility in product applications. |
Both companies have specialized in creating solutions that address the complex demands of today's pharmaceutical and cosmetic industries. BOC Sciences focuses on tailoring its services to assist research in a precise manner, while O'Laughlin Industries showcases its ability to produce a wide range of chemical compounds for various applications. These innovations contribute significantly to the development of new products and technologies in the field.
Conclusion
In conclusion, 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one, also known as Osthol, demonstrates diverse bioactivity and potential in various applications. Its calcium channel blocking properties and promotion of osteogenic differentiation offer promise for therapeutic use.
Furthermore, its fungitoxic effects suggest broader industrial and commercial uses beyond medicine. Safety data combined with recent innovations opens doors for future research and development in this field.
FAQs
1. What is 7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one?
7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one is a compound studied in pharmacology, known to interact with various biological pathways and processes.
2. How does this compound relate to cancer research?
In the context of carcinogenesis, this compound may affect critical pathways such as Akt/mTOR and c-MET. It's also studied in relation to metastatic cancer cells like HCC and LNCaP.
3. Can it influence cell behavior?
Yes, it can impact cell differentiation and apoptosis or programmed cell death. It might also play roles in exocytosis - a process where cells release molecules.
4. Does it have any connection with immune response?
This compound can interact with inflammatory mediators like interleukin, TNF-alpha, and COX-2 which are part of our body’s immune response.
5. Is there any link between this compound and neural activity?
It seems so! This substance could potentially affect acid-sensing ion channels that regulate membrane potential, neurotransmitters like gamma-Aminobutyric acid (GABA), neurotoxins affecting L-type or P/Q-type channels in the hippocampal region of the brain.
6. Are there other notable interactions for this molecule?
The molecule interacts with several biochemical entities including ATP, cyclic nucleotide second messengers, IP3 involved in G protein-coupled receptor (GPCR) signaling pathway; AMPK activation; BMP2 related to bone growth; thromboxane associated with platelets; MMPs involved in degradation processes; histone deacetylase inhibition related to gene regulation.
